What Is the Oxidation Number of O in H2o2
-12 2x 0. The sum of the oxidation numbers of all the atoms in a neutral compound is 0.

The Oxidation Number Of Oxygen In Hydrogen Peroxide Is Youtube
H 2 O 2 has 1 oxidation state of the O-atom.

. If you know that H2O2 is hydrogen peroxide you can immediately assign oxygen the ON -1. As the name implies this compound is a peroxide and thus oxygen will have the oxidation state of minus one. Hydrogen will have the oxidation state of plus one.
In the reaction identify the species undergoing oxidation and reduction. Therefore the product from it will be water in which oxygen has an oxidation number of -2 whereas in H2O2 has an oxidation number of -1 and in O2 the oxygen has an oxidation number of 0. H2O2 has no charge the total oxidation number of the two O atoms must be -2.
The oxidation number of O in compounds is usually -2 but it is -1 in peroxides. The oxidation number of H is 1 Rule 1If you know that H2O2. The oxidation number of hydrogen is 1.
For neutral compounds sum of the oxidation numbers should be zero. If you dont know that. O ΑΣΦ.
We have approached this problem with the concept that the oxidation state of hydrogen peroxide is zero and we know the oxidation number of hydrogen atom that is 1. Mg s 2H₂O g Mg OH₂ H₂ g In an oxidationreduction reaction this is the compound that is reduced. The sum of the oxidation numbers of all the atoms in a neutral compound is 0.
So the oxidation state or oxidation number of oxygen in hydrogen peroxide H_2O_2 is - 1. H2O2 has no charge the total oxidation number of the two O atoms must be -2. -2The oxidation number of O in compounds is usually -2 but it is -1 in peroxides.
Hence in the given question option B is the correct answer that is - 1. Therefore the will have two extra valence electrons eight total. For hydrogen peroxide H 2 O 2 2 1 2 x 0 x -1 Hence the oxidation number of oxygen in H 2 O 2 is -1.
Therefore the product from it will be water in which oxygen has an oxidation number of -2 whereas in H2O2 has an oxidation number of -1 and in O2 the oxygen has an oxidation number of 0. Well to my expert knowledge and my exquisite calculations i think the the negative ion 412 is the key to sucess. The oxidation number of H is 1 For two H atoms the sum is 2.
Therefore oxygen in hydrogen peroxide has actually one less valence electron than oxygen in water. Submit Request Answer Part O2 2 F2 2 OF 2 Express your answer as an integer. The oxidation number of one oxygen atom must be -1.
The oxidation number of H is 1 Rule 1. Of O x. Part B O2 H2 H2O2 Express your answer as an integer.
The oxidation number of. To find the correct oxidation state of O in H2O2 Hydrogen peroxide and each element in the molecule we use a few rules and some simple mathFirst since. If you know that H_2O_2 is hydrogen peroxide you can immediately assign oxygen the ON -1.
The oxidation number of O in is usually -2 but it is -1 in peroxides. What is the oxidation number of oxygen on the reactant side in the following examples. The oxidation state of oxygen in most of the compounds is -2 except in peroxides.
We can confirm this by finding the sum of the oxidation states starting with the two hydrogens present. The number of reactants and products are equal to each other but the atoms change partners. ΔH -982 kJmol ΔS 701 JK mol R 8314 JK mol asked Aug 30 2020 in Chemistry by Jewel.
Hydrogen peroxide has the chemical formula H2O2. H2O2 The oxidation number of H is 1. H2Sg O2g 2Ss 2H2Ol.
Top top the various other hand oxygen in hydrogen peroxide will have one extra valence electron seven total. Hydrogen peroxide H2O2 decomposes according to the equation H2O2 l H2O l 12O2 g. Lets assume the oxidation number of oxygen as x.
The oxidation number of oxygen in compounds is usually -2 but it is -1 in peroxides. The oxidation number of H is 1 Rule 1. Is hydrogen peroxide you can immediately assign oxygen the ON -1.
What is the oxidation number of O in hydrogen peroxide H2O2 Select one. In an oxidationreduction reaction this is the compound that is oxidized. Calculate Kp for this reaction at 25C.
The sum of the oxidation numbers of all the atoms in a neutral compound is 0. The oxidation number of O in compounds is usually -2 but it is -1 in peroxides.

Solved What Is The Oxidation Number Of Hydrogen In H2o2 O Chegg Com

N2h4 H2o2 Hno3 H2o Balanced Chemical Equation

Oxidation Number Of O In H2o2 Is

Oxygen Atom Is Assigned An Oxidation Number Of Chemistry Redox Reactions 14328709 Meritnation Com

Oxidation Number Of O In H2o2 Is

Question Video Deducing The Oxidation State Of Oxygen In Hydrogen Peroxide Nagwa
Is H2o2 Both An Oxidizing And Reducing Agent Quora

How To Find The Oxidation Number For O In Of2 Youtube

Role Of Hydrogen Peroxide Iin The Following Reaction Is Respectively I H 2 O 2 O 3 Rarr H 2 O Zo 2 Ii H 2 O 2 Ag 2 Orarr Aag H 2 O O 2

1 What Is The Oxidation Number Of Hydrogen In H2o 2 What Is Oxidation Number Of Oxygen In H2o2 Science Chemical Reactions And Equations 14938517 Meritnation Com

Kinetic Data Of H 2 O 2 Oxidation In The Presence Of Iron Ions Download Table

How To Find The Oxidation Number For O In O2f2 Dioxygen Difluroide Youtube
How To Balance Fe Oh 2 H2o2 Fe Oh 3 Youtube

How To Find The Oxidation Number For O In H2o2 Hydrogen Peroxide Youtube
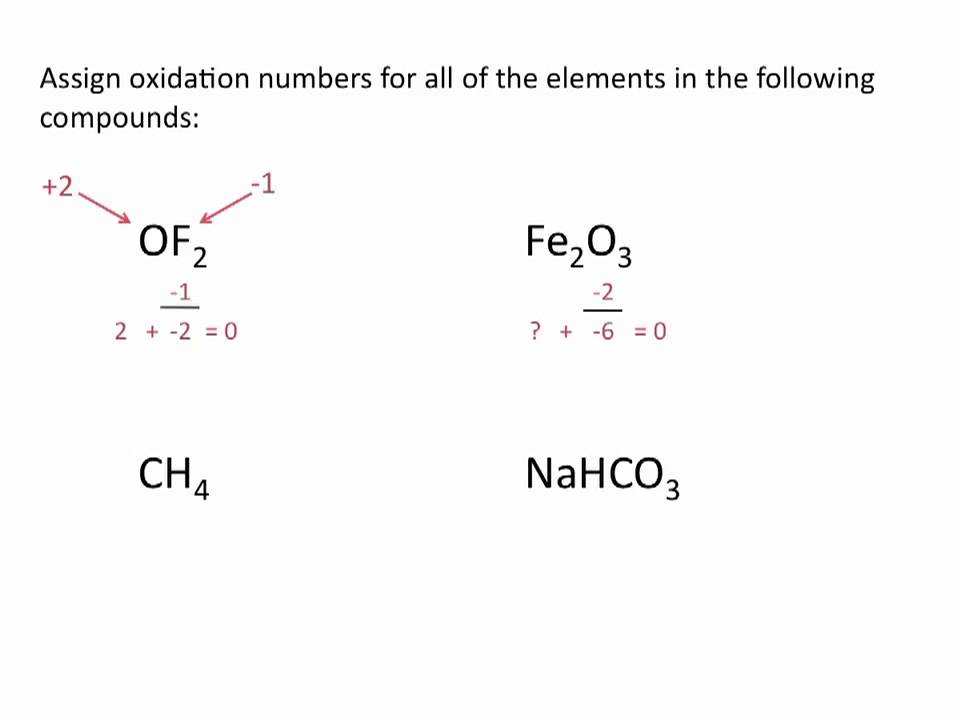
What Are The Oxidation Numbers In The Compound H2o2 Socratic
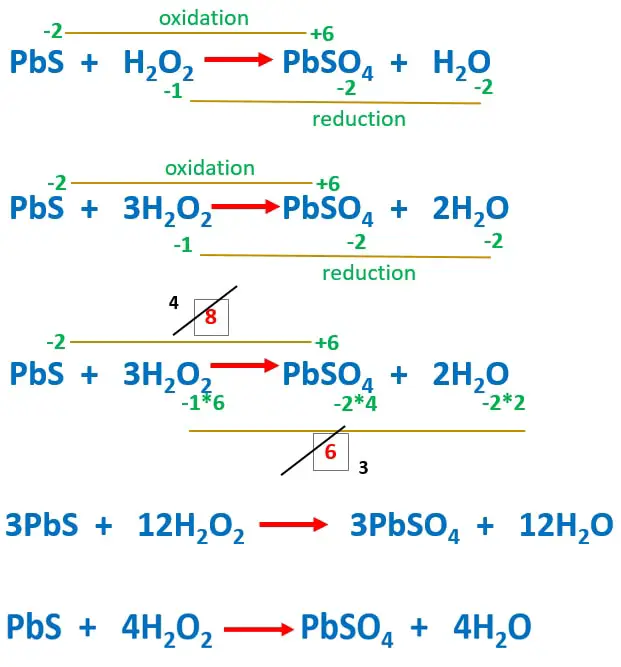
Pbs H2o2 Pbso4 H2o Lead Sulfide Hydrogen Peroxide Reaction
How Will You Show That H2o2 Acts As Both Oxidising And Reducing Agent What Is Meant By 30 Volume Of H2o2 Quora

How To Find The Oxidation Number For O In O2 2 Peroxide Ion Youtube

Comments
Post a Comment